
Chemistry, 09.08.2019 21:30 caityi9619
If the specific heat capacity of water is 4.18 j/g·oc , how much of heat energy is needed to raise the temperature of 10.00 g of liquid water from 14.1 oc to 16.2 oc?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
If the specific heat capacity of water is 4.18 j/g·oc , how much of heat energy is needed to raise t...
Questions

Arts, 06.09.2019 22:30

Biology, 06.09.2019 22:30




Mathematics, 06.09.2019 22:30


History, 06.09.2019 22:30

English, 06.09.2019 22:30



Mathematics, 06.09.2019 22:30



German, 06.09.2019 22:30


Mathematics, 06.09.2019 22:30

Mathematics, 06.09.2019 22:30


Mathematics, 06.09.2019 22:30

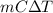
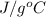
 = change in temperature
= change in temperature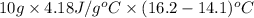
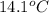 to
to 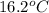 is 87.78 J.
is 87.78 J.

