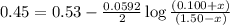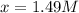Chemistry, 09.08.2019 21:20 thatcurlysophia
77. a voltaic cell consists of a zn/zn2+ half-cell and a ni/ni2+ half-cell at 25°c. the initial concentrations of ni2+ and zn2+ are 1.50 m and 0.100 m, respectively. a. what is the initial cell potential? b. what is the cell potential when the concentration of ni2+ has fallen to 0.500 m? c. what are the concentrations of ni2+ and zn2+ when the cell potential falls to 0.45 v?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
77. a voltaic cell consists of a zn/zn2+ half-cell and a ni/ni2+ half-cell at 25°c. the initial conc...
Questions

Mathematics, 21.05.2021 01:10

Mathematics, 21.05.2021 01:10


Mathematics, 21.05.2021 01:10

Mathematics, 21.05.2021 01:10



Mathematics, 21.05.2021 01:10

History, 21.05.2021 01:10



Arts, 21.05.2021 01:10

Mathematics, 21.05.2021 01:10

Social Studies, 21.05.2021 01:10

Arts, 21.05.2021 01:10


Geography, 21.05.2021 01:10

English, 21.05.2021 01:10

Social Studies, 21.05.2021 01:10

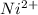 has fallen to 0.500 M is, 0.52 V
has fallen to 0.500 M is, 0.52 V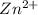 when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M
when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M![E^0_{[Ni^{2+}/Ni]}=-0.23V](/tpl/images/0173/8654/be6be.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0173/8654/4cd18.png)
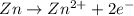
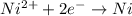
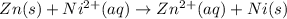
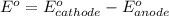
![E^o=E^o_{[Ni^{2+}/Ni]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0173/8654/7d468.png)
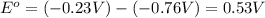
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]}{[Ni^{2+}]}](/tpl/images/0173/8654/c02a9.png)
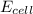 = emf of the cell = ?
= emf of the cell = ?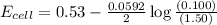
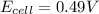
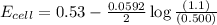
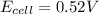
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}+x]}{[Ni^{2+}-x]}](/tpl/images/0173/8654/e92ff.png)