
Chemistry, 09.08.2019 06:10 alannaamarriee
"the combustion of ethylene proceeds by the reaction c2h4(g) + 3o2(g) → 2co2(g) + 2h2o(g) when the rate of disappearance of o2 is 0.23 m s-1, the rate of disappearance of c2h4 is m s-1."

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
"the combustion of ethylene proceeds by the reaction c2h4(g) + 3o2(g) → 2co2(g) + 2h2o(g) when the r...
Questions


Mathematics, 28.01.2020 01:31

Health, 28.01.2020 01:31


Social Studies, 28.01.2020 01:31




Mathematics, 28.01.2020 01:31

Mathematics, 28.01.2020 01:31


Biology, 28.01.2020 01:31

Chemistry, 28.01.2020 01:31



History, 28.01.2020 01:31


Computers and Technology, 28.01.2020 01:31


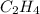 is 0.0766 M/s.
is 0.0766 M/s.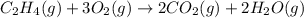
![R=-\frac{1}{1}\frac{d[C_2H_4]}{dt}=-\frac{1}{3}\frac{d[O_2]}{dt}](/tpl/images/0173/6661/4aaec.png)
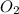 =0.23 M/s
=0.23 M/s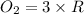
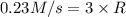
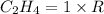
![\frac{d[C_2H_4]}{dt}=1\times 0.23 m/s=0.0766 M/s](/tpl/images/0173/6661/22dea.png)


