
Chemistry, 29.01.2020 20:53 winchester729
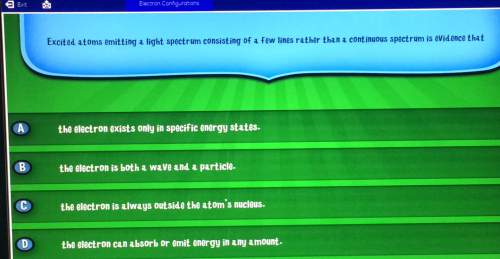
Electron configurationsexitexcited atoms emitting a light spectrum consisting of a few lines rather than a continuous spectrum is evidence thatcad the electron exists only in specific energy statesb the electron is both a wave and a particleco the electron is always outside the atom's nucleus. d the electron can absorb or emit energy in any amount

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3


Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2
You know the right answer?
Electron configurationsexitexcited atoms emitting a light spectrum consisting of a few lines rather...
Questions

Social Studies, 02.10.2021 21:00


Computers and Technology, 02.10.2021 21:00


English, 02.10.2021 21:00


Biology, 02.10.2021 21:00



Chemistry, 02.10.2021 21:10


Social Studies, 02.10.2021 21:10


Mathematics, 02.10.2021 21:10

Social Studies, 02.10.2021 21:10


Mathematics, 02.10.2021 21:10






