
Chemistry, 09.08.2019 04:10 2023brewerantonio
Aseries of chemicals were added to some agno3(aq). nacl(aq) was added first to the silver nitrate solution to produce a precipitate. nh3(aq) was then added to produce a clear solution. hno3(aq) was added last to result in a precipitate. write a balanced net ionic equation for each of the three steps.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

You know the right answer?
Aseries of chemicals were added to some agno3(aq). nacl(aq) was added first to the silver nitrate so...
Questions

Mathematics, 05.10.2019 21:00

Mathematics, 05.10.2019 21:00


History, 05.10.2019 21:00

Mathematics, 05.10.2019 21:00







Mathematics, 05.10.2019 21:00

Physics, 05.10.2019 21:00


Social Studies, 05.10.2019 21:00



Mathematics, 05.10.2019 21:00


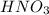 .
.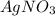 forms an insoluble precipitate of AgCl.
forms an insoluble precipitate of AgCl.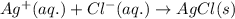
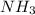 , AgCl gets dissolved into solution due to formation of soluble
, AgCl gets dissolved into solution due to formation of soluble ![[Ag(NH_{3})_{2}]^{+}](/tpl/images/0173/6086/7645c.png) complex.
complex.![AgCl(s)+2NH_{3}(aq.)\rightarrow [Ag(NH_{3})_{2}]^{+}(aq.)+ Cl^{-}(aq.)](/tpl/images/0173/6086/b00b4.png)
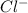 again reacts with free
again reacts with free 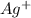 to produce insoluble AgCl
to produce insoluble AgCl![[Ag(NH_{3})_{2}]^{+}(aq.)+2H^{+}(aq.)+Cl^{-}(aq.)\rightarrow AgCl(s)+2NH_{4}^{+}(aq.)](/tpl/images/0173/6086/fabec.png)


