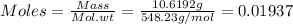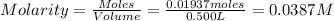Astandard solution is prepared by dissolving 10.6192 g of (nh4)2ce(no3)6 (548.23 g•mol-1, 98.75% purity) in dilute sulfuric acid. the resulting solution is quantitatively transferred to a 500.0-ml volumetric flask and diluted to the mark. what is the ce concentration in the final solution?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
Astandard solution is prepared by dissolving 10.6192 g of (nh4)2ce(no3)6 (548.23 g•mol-1, 98.75% pur...
Questions






Biology, 21.03.2021 19:10

Mathematics, 21.03.2021 19:10

Mathematics, 21.03.2021 19:10

Mathematics, 21.03.2021 19:10


English, 21.03.2021 19:10






Mathematics, 21.03.2021 19:10

Mathematics, 21.03.2021 19:10


Social Studies, 21.03.2021 19:10