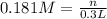Chemistry, 08.08.2019 06:20 jsharma57p7enrw
In the laboratory, a student adds 19.7 g of barium acetate to a 500. ml volumetric flask and adds water to the mark on the neck of the flask. calculate the concentration (in mol/l) of barium acetate, the barium ion and the acetate ion in the solution. [ba(ch3coo)2] = m [ba2+] = m [ch3coo-] = m
calculate the mass, in grams, of iron(ii) sulfate that must be added to a 300-ml volumetric flask in order to prepare 300 ml of a 0.181 m aqueous solution of the salt.
grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

You know the right answer?
In the laboratory, a student adds 19.7 g of barium acetate to a 500. ml volumetric flask and adds wa...
Questions


Spanish, 14.01.2020 23:31

Biology, 14.01.2020 23:31


Physics, 14.01.2020 23:31



English, 14.01.2020 23:31


Mathematics, 14.01.2020 23:31




Mathematics, 14.01.2020 23:31

Mathematics, 14.01.2020 23:31

Advanced Placement (AP), 14.01.2020 23:31

English, 14.01.2020 23:31


English, 14.01.2020 23:31

![[Ba(CH_3COO)_2]=0.1545 mol/L](/tpl/images/0173/1748/733d1.png)
![[Ba^{2+}]=0.1545 mol/L](/tpl/images/0173/1748/704db.png)
![[CH_3COO^-]=0.3090 mol/L](/tpl/images/0173/1748/1e1ae.png)
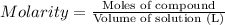
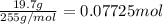
![[Ba(CH_3COO)_2]=\frac{0.07725 mol}{0.5L}=0.1545 mol/L](/tpl/images/0173/1748/ea20e.png)
![[Ba^{2+}]=1\times [Ba(CH_3COO)_2]](/tpl/images/0173/1748/fd1e3.png)
![[Ba^{2+}]=1\times 0.1545 mol/L=0.1545 mol/L](/tpl/images/0173/1748/1f46a.png)
![[CH_3COO^-]=2\times [Ba(CH_3COO)_2]](/tpl/images/0173/1748/6fb75.png)
![[CH_3COO^-]=2\times 0.1545 mol/l=0.3090 mol/L](/tpl/images/0173/1748/e3fff.png)
![[Fe_2(SO_4)_3]=0.181 M](/tpl/images/0173/1748/b830a.png)