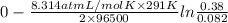Aconcentration cell is built based on the following half reactions by using two pieces of zinc as electrodes, two zn2+ solutions, 0.129 m and 0.427 m, and all other materials needed for a galvanic cell. what will the potential of this cell be when the cathode concentration of zn2+ has changed by 0.047 m at 291 k?
zn2+ + 2 e- ? zn eo = -0.761 v

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

You know the right answer?
Aconcentration cell is built based on the following half reactions by using two pieces of zinc as el...
Questions




















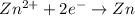 ,
, 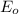 = -0.761 V
= -0.761 V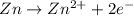 ,
, 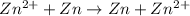
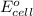 for the given reaction is zero.
for the given reaction is zero.![E^{o}_{cell} - \frac{RT}{nF} ln \frac{[Zn^{2+}]_{products}}{[Zn^{2+}]_{reactants}}](/tpl/images/0173/1699/4d9c9.png)