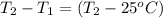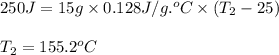Chemistry, 08.08.2019 05:30 jhashknkughb6759
A15.0 g metal sample at 25.0 °c has 250 j of heat added to it. the specific heat of the metal is 0.128 j/g.°c. what is the final temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
A15.0 g metal sample at 25.0 °c has 250 j of heat added to it. the specific heat of the metal is 0.1...
Questions

Mathematics, 11.06.2021 18:20







Mathematics, 11.06.2021 18:20

Biology, 11.06.2021 18:20



Computers and Technology, 11.06.2021 18:20


English, 11.06.2021 18:20


Chemistry, 11.06.2021 18:20




History, 11.06.2021 18:20

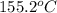
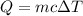
 = change in temperature =
= change in temperature =