
Chemistry, 08.08.2019 04:20 vipergod07
Carbon dioxide (co2) is an abundant greenhouse gas that is present in high atmospheric concentrations as a result of increasing air pollution. the two processes for the natural removal of co2 from the atmosphere are photosynthesis and dissolution into the oceans. complete the following chemical equation for the dissolution of co2 in water. remember to include the physical states of the products. co. lag) +h what happens to the ph of the ocean as more co2 dissolves? oincreases o decreases o no change

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

You know the right answer?
Carbon dioxide (co2) is an abundant greenhouse gas that is present in high atmospheric concentration...
Questions

Mathematics, 27.09.2021 17:10


Computers and Technology, 27.09.2021 17:10

Social Studies, 27.09.2021 17:10

History, 27.09.2021 17:10


English, 27.09.2021 17:10

Mathematics, 27.09.2021 17:10



Physics, 27.09.2021 17:10

Biology, 27.09.2021 17:10

Social Studies, 27.09.2021 17:10

Physics, 27.09.2021 17:10

Mathematics, 27.09.2021 17:20

Mathematics, 27.09.2021 17:20


Mathematics, 27.09.2021 17:20


English, 27.09.2021 17:20

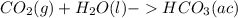 ; The pH decrease.
; The pH decrease.

