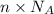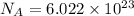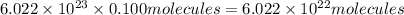Chemistry, 08.08.2019 02:10 lizredrose5
0. in 0.100 mole of dimethylhydrazine, (ch3)2n2h2, there are a. 6.02 x 1023 molecules b. 6.02 x 1024 molecules c. 7.22 x 1023 atoms d. 6.62 x 1023 atoms e. 4.82 x 1023 atoms

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
0. in 0.100 mole of dimethylhydrazine, (ch3)2n2h2, there are a. 6.02 x 1023 molecules b. 6.02 x 1024...
Questions



Physics, 21.10.2019 21:00

Biology, 21.10.2019 21:10




History, 21.10.2019 21:10





English, 21.10.2019 21:10


Mathematics, 21.10.2019 21:10

Mathematics, 21.10.2019 21:10




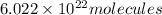 of dimethylhydrazine are present in 0.100 mol.
of dimethylhydrazine are present in 0.100 mol.