
Chemistry, 07.08.2019 23:30 kiaraangely100400
Write the complete neutralization reaction between hcl and koh in aqueous solution. if we add .01 mol koh to .03 mol hcl in 1 l h2o, what is the ph of the resulting solution, and is it acidic or basic?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Write the complete neutralization reaction between hcl and koh in aqueous solution. if we add .01 mo...
Questions

Business, 01.12.2020 20:10



Business, 01.12.2020 20:10



Mathematics, 01.12.2020 20:10

Mathematics, 01.12.2020 20:10

English, 01.12.2020 20:10

Mathematics, 01.12.2020 20:10


Mathematics, 01.12.2020 20:10

English, 01.12.2020 20:10


Mathematics, 01.12.2020 20:10


Mathematics, 01.12.2020 20:10

Mathematics, 01.12.2020 20:10


English, 01.12.2020 20:10

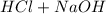 →
→ 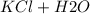
![pH=-log[H+]](/tpl/images/0172/9419/16a9e.png)
![pH=-log[0.02]=1.69897](/tpl/images/0172/9419/d9650.png)



