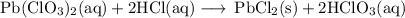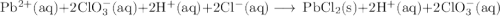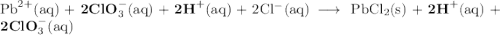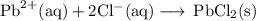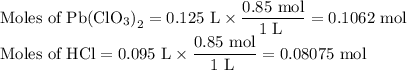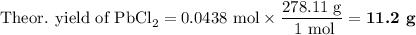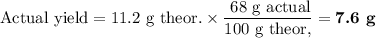Chemistry, 07.08.2019 04:10 kraigstlistt
Lead all chlorate is mixed with hydrolylic acid. each solution is 0.85 molar. write balanced, molecular, ionic, and net equations with state labels. use solubility rules and knowledge of strong acids. predict how many grams of what solid product can be collected if 125ml lead ll chlorate was treated with with 95 ml of the hydrologic acid. if percent yield was 68 percent how much product was collected and what is the molarity of the chlorate? what is the final concentration of pb2+. show work!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Lead all chlorate is mixed with hydrolylic acid. each solution is 0.85 molar. write balanced, molecu...
Questions

Chemistry, 07.10.2019 22:30

Spanish, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

English, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

English, 07.10.2019 22:30


Mathematics, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

Mathematics, 07.10.2019 22:30

Social Studies, 07.10.2019 22:30


History, 07.10.2019 22:30



Mathematics, 07.10.2019 22:30