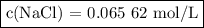Chemistry, 07.08.2019 01:10 BrainlyAvenger
To study the effect of dissolved salt on the rusting of an iron sample, a student prepared a solution of nacl by dissolving 3.038 g of nacl in enough
water to make 792.2 ml of solution. what is the molarity of this solution?
c(nacl)=

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
To study the effect of dissolved salt on the rusting of an iron sample, a student prepared a solutio...
Questions


History, 06.10.2019 10:02





Mathematics, 06.10.2019 10:02






Mathematics, 06.10.2019 10:02

Mathematics, 06.10.2019 10:02

English, 06.10.2019 10:02


Social Studies, 06.10.2019 10:02

Mathematics, 06.10.2019 10:02

Health, 06.10.2019 10:02