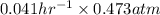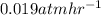Chemistry, 07.08.2019 00:10 KindaSmartPersonn
The breakdown of a certain pollutant x in sunlight is known to follow first-order kinetics. an atmospheric scientist studying the process fills a 20.0lreaction flask with a sample of urban air and finds that the partial pressure of x in the flask decreases from 0.473atm to 0.376atm over 5.6hours.
calculate the initial rate of decomposition of x, that is, the rate at which xwas disappearing at the start of the experiment.
round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
The breakdown of a certain pollutant x in sunlight is known to follow first-order kinetics. an atmos...
Questions

Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57


Mathematics, 09.06.2020 02:57


Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57




Biology, 09.06.2020 02:57



Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57

Computers and Technology, 09.06.2020 02:57

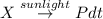
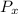
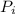 = initial partial pressure of X = 0.473 atm
= initial partial pressure of X = 0.473 atm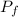 = final partial pressure of X = 0.376 atm
= final partial pressure of X = 0.376 atm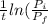
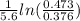
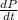 = k
= k