
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the following concentrations are present: [so2] = 0.010 m; [so3] = 10.m; [o2] = 0.010 m. is the mixture at equilibrium? if not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium? yes, the mixture is at equilibrium. no, left to right. no, right to left. there is not enough information to be able to predict the direction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the fo...
Questions

Mathematics, 17.10.2019 20:30


Mathematics, 17.10.2019 20:30


Mathematics, 17.10.2019 20:30

Mathematics, 17.10.2019 20:30



Mathematics, 17.10.2019 20:30




Mathematics, 17.10.2019 20:30




English, 17.10.2019 20:30

Spanish, 17.10.2019 20:30

Biology, 17.10.2019 20:30

Computers and Technology, 17.10.2019 20:30


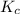 = 4.3 \times 10^{6}[/tex].
= 4.3 \times 10^{6}[/tex].![[SO_2]](/tpl/images/0172/4735/0303a.png) = 0.010 M;
= 0.010 M; ![[SO_3]](/tpl/images/0172/4735/8a06f.png) = 10.M;
= 10.M; ![[O_2]](/tpl/images/0172/4735/b0db0.png) = 0.010 M.
= 0.010 M.![\frac{[SO_{3}]^{2}}{[SO_{2}]^{2}[O_{2}]}](/tpl/images/0172/4735/2750c.png)

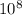
 , then reaction moves in the backward direction.
, then reaction moves in the backward direction.

