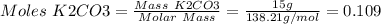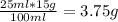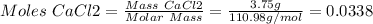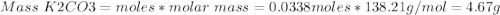Chemistry, 06.08.2019 19:30 santosbeti90
1. write a balanced equation for the precipitation of calcium carbonate from potassium carbonate and calcium chloride. 2. using this balanced equation, determine the limiting reactant if 15 grams of calcium chloride was reacted with 15 grams of potassium carbonate. 3. using your answer for question 2, determine the mass of potassium carbonate needed to fully precipitate all the calcium from a 25 ml sample of 15% calcium chloride.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
1. write a balanced equation for the precipitation of calcium carbonate from potassium carbonate and...
Questions

Mathematics, 15.07.2019 03:30


Chemistry, 15.07.2019 03:30

Mathematics, 15.07.2019 03:30

History, 15.07.2019 03:30

Computers and Technology, 15.07.2019 03:30

Computers and Technology, 15.07.2019 03:30


History, 15.07.2019 03:30

Computers and Technology, 15.07.2019 03:30

Biology, 15.07.2019 03:30

Computers and Technology, 15.07.2019 03:30



Social Studies, 15.07.2019 03:30


Mathematics, 15.07.2019 03:30


Mathematics, 15.07.2019 03:30

Computers and Technology, 15.07.2019 03:30

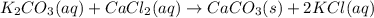
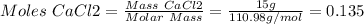
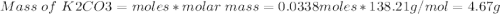 = 15 g
= 15 g