
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient (q) is greater than k, reactant must be converted to products to reach equilibrium.2. for a chemical system at equilibrium, the forward and reverse rates of reaction are equal.3. for a chemical system at equilibrium, the concentrations of products divided by the concentrations of reactants equals one.1 only2 only3 only1 and 21, 2, and 3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient...
Questions



Mathematics, 23.03.2021 20:30

Mathematics, 23.03.2021 20:30

English, 23.03.2021 20:30

Mathematics, 23.03.2021 20:30



History, 23.03.2021 20:30

English, 23.03.2021 20:30

Mathematics, 23.03.2021 20:30


Social Studies, 23.03.2021 20:30



English, 23.03.2021 20:30

Mathematics, 23.03.2021 20:30

Social Studies, 23.03.2021 20:30


Mathematics, 23.03.2021 20:30

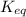 , then it means that the reaction is proceeding in the backward reaction. Whereas if Q <
, then it means that the reaction is proceeding in the backward reaction. Whereas if Q < 

