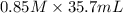Chemistry, 06.08.2019 06:10 vlactawhalm29
You have 185 ml of an acid with an unknown concentration. to determine the concentration you decide to use a 0.85 m solution of sodium hydroxide to titrate the acid. after the titration you determined that it took 35.7 ml of the 0.85 m solution of sodium hydroxid to reach the endpoint. what is the acids concentration?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
You have 185 ml of an acid with an unknown concentration. to determine the concentration you decide...
Questions

English, 09.06.2021 09:10

Mathematics, 09.06.2021 09:10

Mathematics, 09.06.2021 09:10

Mathematics, 09.06.2021 09:10

Mathematics, 09.06.2021 09:10

Health, 09.06.2021 09:10

Biology, 09.06.2021 09:10

History, 09.06.2021 09:10

Biology, 09.06.2021 09:10

Health, 09.06.2021 09:10





Physics, 09.06.2021 09:10

Biology, 09.06.2021 09:10


English, 09.06.2021 09:10

English, 09.06.2021 09:10

Mathematics, 09.06.2021 09:10

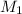 = ?,
= ?, 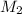 = 0.85 M
= 0.85 M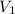 = 185 mL,
= 185 mL, 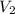 = 35.7 mL
= 35.7 mL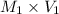 =
= 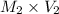
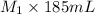 =
=