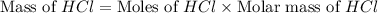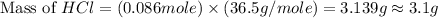Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30.0 g of hcl (fw = 36.5 g/mol) react according to the following chemical equation? mno2 + 4 hcl ® mncl2 + cl2 + 2 h2o3.1 g hcl23.3 g hcl4.02 g mno28.0 g mno212.1 g mno2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30...
Questions


Physics, 13.11.2020 21:00

Mathematics, 13.11.2020 21:10




Chemistry, 13.11.2020 21:10

Mathematics, 13.11.2020 21:10

History, 13.11.2020 21:10



Physics, 13.11.2020 21:10

Health, 13.11.2020 21:10




Social Studies, 13.11.2020 21:10

Mathematics, 13.11.2020 21:10


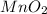 = 16.0 g
= 16.0 g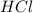 = 30.0 g
= 30.0 g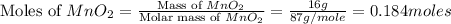
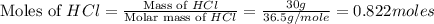
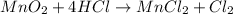
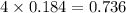 moles of
moles of