Chemistry, 06.08.2019 05:20 tykiabrown8111
Which one of the following statements is true about the equilibrium constant for a reaction if δg° for the reaction is negative? k > 1 k < 1 k = 1 k = 0

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1


Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
Which one of the following statements is true about the equilibrium constant for a reaction if δg° f...
Questions



Health, 16.09.2019 15:30


Mathematics, 16.09.2019 15:30

Mathematics, 16.09.2019 15:30



Social Studies, 16.09.2019 15:30




Mathematics, 16.09.2019 15:30



Biology, 16.09.2019 15:30




Computers and Technology, 16.09.2019 15:30

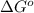 for the reaction is negative.
for the reaction is negative.