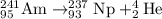Chemistry, 06.08.2019 04:20 fangirl2837
There are several technological applications for the transuranium elements (z > 92). an important one is in smoke detectors, which can use the decay of a tiny amount of americium-241 to neptunium-237. what subatomic particle is emitted from that decay process? explain your answer.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
There are several technological applications for the transuranium elements (z > 92). an importan...
Questions



English, 18.11.2020 02:00


Mathematics, 18.11.2020 02:00


History, 18.11.2020 02:00







Spanish, 18.11.2020 02:00


Mathematics, 18.11.2020 02:00



Mathematics, 18.11.2020 02:00