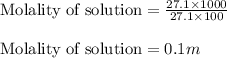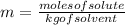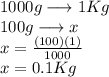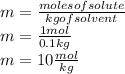Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 23.06.2019 10:00
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1

Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
You know the right answer?
If you combine 27.1 g of a solute that has a molar mass of 27.1 g/mol with 100.0 g of a solvent, wha...
Questions




Mathematics, 23.10.2020 16:10




Mathematics, 23.10.2020 16:10






Mathematics, 23.10.2020 16:10






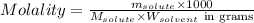
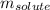 = Given mass of solute = 27.1 g
= Given mass of solute = 27.1 g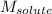 = Molar mass of solute = 27.1 g/mol
= Molar mass of solute = 27.1 g/mol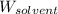 = Mass of solvent = 100 g
= Mass of solvent = 100 g