
Chemistry, 06.08.2019 02:30 jayjay9434
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the reaction starts with a mixture of pcl5, pcl3, and cl2 at pressures 0.177 atm, 0.223 atm, and 0.111 atm, respectively, at 250ºc. when the mixture comes to equilibrium at that temperature, which pressures will have decreased and which will have increased? explain why.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

You know the right answer?
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the re...
Questions




Medicine, 31.08.2020 02:01





Physics, 31.08.2020 02:01




Computers and Technology, 31.08.2020 02:01



Mathematics, 31.08.2020 02:01




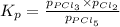
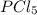 ,
,  , and
, and 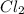 at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.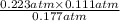
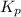 > 0.14 atm. As calculated value is less than the given value of
> 0.14 atm. As calculated value is less than the given value of 

