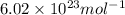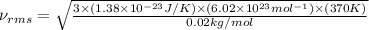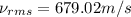Chemistry, 06.08.2019 02:20 mlittleduck6947
Consider neon, a noble gas whose molecules consist of single atoms of atomic mass 0.02 kg/mol. what is the average kinetic energy of a neon atom when the gas is at a temperature of 370 k? avogadro’s number is 6.02 × 1023 mol−1 and boltzmann’s constant is 1.38 × 10−23 j/k. answer in units of j. question 5, chap 19, sect 4. part 2 of 3 10 points what is the root mean square speed of a neon atom under such conditions? answer in units of m/s.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Consider neon, a noble gas whose molecules consist of single atoms of atomic mass 0.02 kg/mol. what...
Questions

History, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50

History, 21.07.2019 19:50


Mathematics, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50

Biology, 21.07.2019 19:50



English, 21.07.2019 19:50


Mathematics, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50

Mathematics, 21.07.2019 19:50


Mathematics, 21.07.2019 19:50

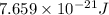
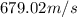
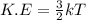
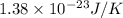
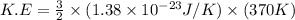
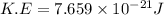
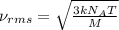
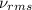 = root mean square speed
= root mean square speed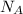 = Avogadro’s number =
= Avogadro’s number =