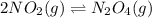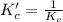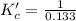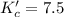The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625 mole of n2o4 was introduced into a 5.00 l vessel and was allowed to decompose until equilibrium was reached. the concentration of n2o4 at equilibrium was 0.0750 m. calculate kc for the reaction.0.0500.07500.100.1257.5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625...
Questions

Biology, 07.12.2019 19:31




Mathematics, 07.12.2019 20:31


History, 07.12.2019 20:31




English, 07.12.2019 20:31

Spanish, 07.12.2019 20:31



English, 07.12.2019 20:31



Mathematics, 07.12.2019 20:31

Mathematics, 07.12.2019 20:31

Mathematics, 07.12.2019 20:31

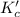 for the reaction is, 7.5
for the reaction is, 7.5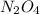 =
= 
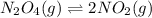
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0172/0236/271f5.png)
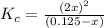
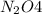 at equilibrium = 0.0750 M
at equilibrium = 0.0750 M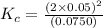
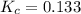
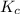 for the given reaction.
for the given reaction.