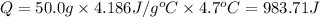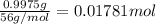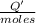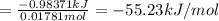Chemistry, 06.08.2019 01:20 larissacrystalow8g2w
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperature is 22.3 degrees celsius. after addition of the solid, the solution temperature is raised to about 27.0 degrees celsius. the substance is known to have a molar mass of about 56 g/mol. calculate the enthlapy of solution in kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
0.9775 grams of an unknown compound is dissolved in 50.0 ml of water. initially the water temperatur...
Questions


Mathematics, 16.01.2020 20:31

Mathematics, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31

Mathematics, 16.01.2020 20:31

Mathematics, 16.01.2020 20:31



Mathematics, 16.01.2020 20:31


Health, 16.01.2020 20:31

History, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31





Biology, 16.01.2020 20:31