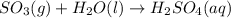Acid rain is produced from co2, and dissolving in atmospheric water molecules. this causes a decrease in the ph of water by increasing the h+ concentration. complete the reaction between sulfur trioxide and water in the formation of acid rain. include the phase of the acid produced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Acid rain is produced from co2, and dissolving in atmospheric water molecules. this causes a decre...
Questions









Mathematics, 06.11.2020 16:20








Biology, 06.11.2020 16:20


Mathematics, 06.11.2020 16:20