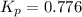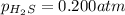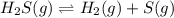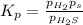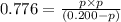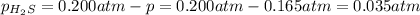Chemistry, 03.08.2019 03:30 kandikisses2101
At a certain temperature, the for the decomposition of h2s is 0.776. h2s(g)↽−−⇀h2(g)+s(g) initially, only h2s is present at a pressure of 0.200 atm in a closed container. what is the total pressure in the container at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
At a certain temperature, the for the decomposition of h2s is 0.776. h2s(g)↽−−⇀h2(g)+s(g) initially...
Questions

Computers and Technology, 15.12.2020 01:00



Spanish, 15.12.2020 01:00

English, 15.12.2020 01:00

History, 15.12.2020 01:00




Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Physics, 15.12.2020 01:00

Social Studies, 15.12.2020 01:00




Mathematics, 15.12.2020 01:00

History, 15.12.2020 01:00