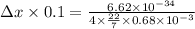Answers: 2


Another question on Chemistry




Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Use the heisenberg uncertainty principle to calculate the uncertainity in meters in the position of...
Questions


History, 15.03.2020 03:01


Mathematics, 15.03.2020 03:01



Geography, 15.03.2020 03:02

Mathematics, 15.03.2020 03:02



Mathematics, 15.03.2020 03:03

Mathematics, 15.03.2020 03:04

Mathematics, 15.03.2020 03:04


Mathematics, 15.03.2020 03:04


Mathematics, 15.03.2020 03:05



Mathematics, 15.03.2020 03:06