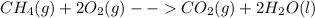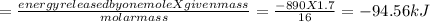Chemistry, 03.08.2019 00:20 jtorres0520
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon dioxide and water: ch4 (g) 2o2 (g) → co2 (g) 2h2o(l) δh = -890.0 kj
calculate the value of q (kj) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
(a) -94.6 kj
(b) 0.0306 kj
(c) -0.0106 kj
(d) 32.7 kj
(e) -9.46 × 10^4 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

You know the right answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon di...
Questions


English, 20.09.2021 14:00

English, 20.09.2021 14:00



Mathematics, 20.09.2021 14:00


Health, 20.09.2021 14:00

Mathematics, 20.09.2021 14:00


Mathematics, 20.09.2021 14:00

Mathematics, 20.09.2021 14:00


Mathematics, 20.09.2021 14:00






Physics, 20.09.2021 14:00