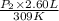Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Consider 4.60 l of a gas at 365 mmhg and 20 c . if the container is compressed to 2.60 l and the tem...
Questions

Biology, 13.11.2019 22:31

Mathematics, 13.11.2019 22:31

Computers and Technology, 13.11.2019 22:31



Biology, 13.11.2019 22:31

Computers and Technology, 13.11.2019 22:31


Mathematics, 13.11.2019 22:31

History, 13.11.2019 22:31

Health, 13.11.2019 22:31

Mathematics, 13.11.2019 22:31

Mathematics, 13.11.2019 22:31


Computers and Technology, 13.11.2019 22:31

Biology, 13.11.2019 22:31

Biology, 13.11.2019 22:31


Mathematics, 13.11.2019 22:31

Chemistry, 13.11.2019 22:31

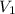 = 4.60 L,
= 4.60 L, 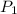 = 365 mm Hg
= 365 mm Hg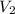 = 2.60 L,
= 2.60 L, 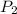 = ?
= ?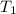 = (20 + 273) K = 293 K,
= (20 + 273) K = 293 K, 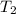 = (36 + 273) K = 309 K
= (36 + 273) K = 309 K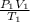 =
= 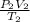
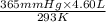 =
=