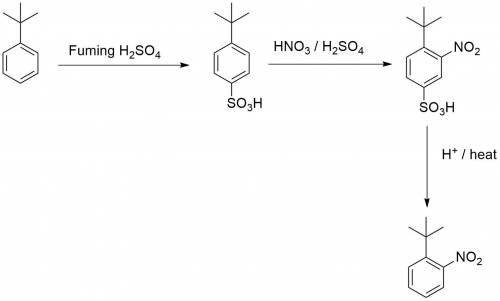
Chemistry, 02.08.2019 23:20 kkeith121p6ujlt
Knowing that the sulfonation of benzenes with sulfuric acid is a reversible process, explain how you could leverage this to allow you to make just 1-(1,1-dimethylethyl)-2-nitrobenzen e from (1,1-dimethylethyl)benzene.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2


Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 15:00
What is the mass in grams of 0.94 moles of sodium bicarbonate, nahco3?
Answers: 1
You know the right answer?
Knowing that the sulfonation of benzenes with sulfuric acid is a reversible process, explain how you...
Questions

Computers and Technology, 06.11.2021 02:20

Geography, 06.11.2021 02:20



History, 06.11.2021 02:20


Spanish, 06.11.2021 02:20





Mathematics, 06.11.2021 02:20

Mathematics, 06.11.2021 02:20




SAT, 06.11.2021 02:20



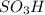 groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.
groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.