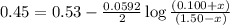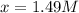Chemistry, 02.08.2019 22:30 meeeekmill
Avoltaic cell consists of a zn> zn2+ half-cell and a ni> ni2+ half-cell at 25 °c. the initial concentrations of ni2+ and zn2+ are 1.50 m and 0.100 m, respectively. a. what is the initial cell potential? b. what is the cell potential when the concentration of ni2+ has fallen to 0.500 m? c. what are the concentrations of ni2+ and zn2+ when the cell potential falls to 0.45 v?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Avoltaic cell consists of a zn> zn2+ half-cell and a ni> ni2+ half-cell at 25 °c. the initial...
Questions

History, 30.01.2020 03:03




Mathematics, 30.01.2020 03:04

Social Studies, 30.01.2020 03:04

Social Studies, 30.01.2020 03:04

History, 30.01.2020 03:04


Mathematics, 30.01.2020 03:04


Mathematics, 30.01.2020 03:04

Mathematics, 30.01.2020 03:04


Advanced Placement (AP), 30.01.2020 03:04

History, 30.01.2020 03:04




Computers and Technology, 30.01.2020 03:04

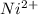 has fallen to 0.500 M is, 0.52 V
has fallen to 0.500 M is, 0.52 V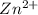 when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M
when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M![E^0_{[Ni^{2+}/Ni]}=-0.23V](/tpl/images/0163/3369/be6be.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0163/3369/4cd18.png)
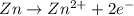
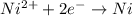
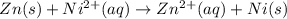
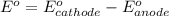
![E^o=E^o_{[Ni^{2+}/Ni]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0163/3369/7d468.png)
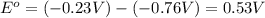
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]}{[Ni^{2+}]}](/tpl/images/0163/3369/c02a9.png)
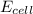 = emf of the cell = ?
= emf of the cell = ?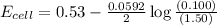
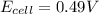
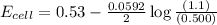
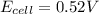
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}+x]}{[Ni^{2+}-x]}](/tpl/images/0163/3369/e92ff.png)