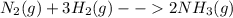Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammon...
Questions


History, 14.04.2021 21:00



English, 14.04.2021 21:00

Mathematics, 14.04.2021 21:00

Social Studies, 14.04.2021 21:00

Chemistry, 14.04.2021 21:00

Mathematics, 14.04.2021 21:00

Mathematics, 14.04.2021 21:00


Mathematics, 14.04.2021 21:00


Geography, 14.04.2021 21:00



Mathematics, 14.04.2021 21:00


Mathematics, 14.04.2021 21:00

Mathematics, 14.04.2021 21:00