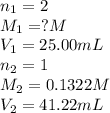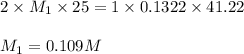Chemistry, 02.08.2019 21:40 arianabarber
A25.00-ml sample of an h2so4 solution of unknown concentration is titrated with a 0.1322 m koh solution. a volume of 41.22 ml of koh is required to reach the equivalence point. what is the concentration of the unknown h2so4 solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

You know the right answer?
A25.00-ml sample of an h2so4 solution of unknown concentration is titrated with a 0.1322 m koh solut...
Questions


Social Studies, 08.12.2020 22:00


Mathematics, 08.12.2020 22:00


Chemistry, 08.12.2020 22:00


Biology, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00

Chemistry, 08.12.2020 22:00









Mathematics, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00

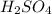 comes out to be 0.109 M.
comes out to be 0.109 M.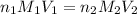
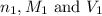 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 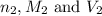 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.