Chemistry, 02.08.2019 20:30 therealpr1metime45
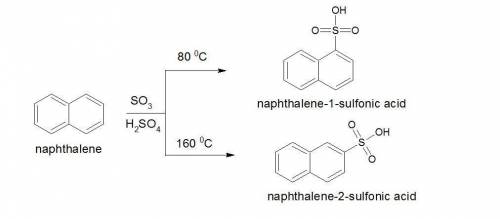
Sulfonation of naphthalene, c10h8, results in two products. one product is kinetically favored and predominates in the beginning of the reaction. because the reaction is reversible, eventually the kinetically slower but thermodynamically favored product predominates. draw the structure of these two products. (the naphthalene ring is already drawn for you. do not change the double bond configuration in the given structures.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 15:00
In two or more complete sentences describe all of the van der waals forces that exist between molecules of sulfur dioxide, so2.
Answers: 1
You know the right answer?
Sulfonation of naphthalene, c10h8, results in two products. one product is kinetically favored and p...
Questions


Mathematics, 02.10.2020 18:01


Mathematics, 02.10.2020 18:01

Mathematics, 02.10.2020 18:01


Mathematics, 02.10.2020 18:01



History, 02.10.2020 18:01

Physics, 02.10.2020 18:01

Mathematics, 02.10.2020 18:01

Biology, 02.10.2020 18:01

History, 02.10.2020 18:01


Mathematics, 02.10.2020 18:01




Mathematics, 02.10.2020 18:01