
Chemistry, 02.08.2019 20:30 LeoValdez5782
Suppose a layer of oil is on the top of a beaker of water. water in many oils is slightly soluble, so its concentration is so low that we can treat it as an ideal solute. 1) suppose that at 283 k, the equilibrium concentration is 9 × 10-4 water molecules per oil molecule, and it takes 2.208 × 10-20 j to transfer one water molecule into the oil. what is the equilibrium concentration at 293 k, assuming that nothing else changes

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

You know the right answer?
Suppose a layer of oil is on the top of a beaker of water. water in many oils is slightly soluble, s...
Questions

Business, 13.07.2019 11:30








French, 13.07.2019 11:30

History, 13.07.2019 11:30

Mathematics, 13.07.2019 11:30


Mathematics, 13.07.2019 11:30


Mathematics, 13.07.2019 11:30

History, 13.07.2019 11:30



Mathematics, 13.07.2019 11:30

Computers and Technology, 13.07.2019 11:30

 J/molecule
J/molecule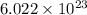 atoms.
atoms.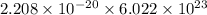 J/mol
J/mol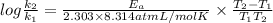
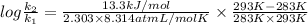
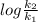 = 0.08377
= 0.08377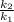 = 1.213 =
= 1.213 = 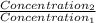
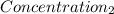 =
= 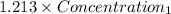
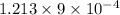
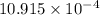 water molecules per oil molecule
water molecules per oil molecule

