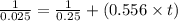Chemistry, 01.08.2019 06:10 lilytimpsonx
The decomposition of nobr follows second order kinetics. the rate constant is found to be 0.556 m-1 s-1. if the initial concentration of nobr in the container is 0.25 m, how long will it take for the concentration to decrease to 0.025 m?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
The decomposition of nobr follows second order kinetics. the rate constant is found to be 0.556 m-1...
Questions


Biology, 25.03.2020 00:09


Mathematics, 25.03.2020 00:09



Biology, 25.03.2020 00:09

Social Studies, 25.03.2020 00:09


Mathematics, 25.03.2020 00:09


Mathematics, 25.03.2020 00:09

Chemistry, 25.03.2020 00:09

Biology, 25.03.2020 00:09

Social Studies, 25.03.2020 00:09


English, 25.03.2020 00:09



![\frac{1}{[NOBr]}=\frac{1}{[NOBr]_{0}}+kt](/tpl/images/0157/1905/03ea7.png)
![[NOBr]_{0}](/tpl/images/0157/1905/349bd.png) is initial concentration of NOBr and k is rate constant
is initial concentration of NOBr and k is rate constant and
and