
Chemistry, 01.08.2019 06:10 amykookie24
1. write the reaction rate expressions for the following reactions in terms of the disappearance of the reactants and the appearance of products: (6 points) a. h2(g) + i2(g) → 2hi(g) b. 5br–(aq) + bro3–(aq) + 6h+(aq) → 3br2(aq) + 3h2o(l)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
1. write the reaction rate expressions for the following reactions in terms of the disappearance of...
Questions



History, 22.09.2019 07:20



Biology, 22.09.2019 07:20


Mathematics, 22.09.2019 07:20

SAT, 22.09.2019 07:20





Mathematics, 22.09.2019 07:20


Mathematics, 22.09.2019 07:20

Biology, 22.09.2019 07:20



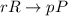
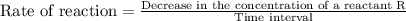
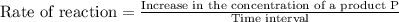
![\text{Rate of reaction}=-\frac{1}{r}\frac{\Delta [R]}{\Delta t}=+\frac{1}{p}\frac{\Delta [P]}{\Delta t}](/tpl/images/0157/1902/6c23f.png)
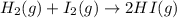
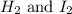 is given as:
is given as:![\text{Rate of disappearance of }H_2=-\frac{\Delta [H_2]}{\Delta t}](/tpl/images/0157/1902/a0e34.png)
![\text{Rate of disappearance of }I_2=-\frac{\Delta [I_2]}{\Delta t}](/tpl/images/0157/1902/5866d.png)
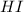 is given as:
is given as:![\text{Rate of appearance of HI}=+\frac{1}{2} \frac{\Delta [HI]}{\Delta t}](/tpl/images/0157/1902/c014e.png)
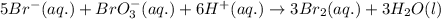
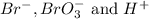 is given as:
is given as:![\text{Rate of disappearance of }Br^-=-\frac{1}{5} \frac{\Delta [Br^-]}{\Delta t}](/tpl/images/0157/1902/8703b.png)
![\text{Rate of disappearance of }BrO_3^-=-\frac{\Delta [BrO_3^-]}{\Delta t}](/tpl/images/0157/1902/61d70.png)
![\text{Rate of disappearance of }H^+=-\frac{1}{6} \frac{\Delta [H^+]}{\Delta t}](/tpl/images/0157/1902/449ff.png)
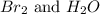 is given as:
is given as:![\text{Rate of appearance of }Br_2=+\frac{1}{3} \frac{\Delta [Br_2]}{\Delta t}](/tpl/images/0157/1902/882fd.png)
![\text{Rate of appearance of }H_2O=+\frac{1}{3} \frac{\Delta [H_2O]}{\Delta t}](/tpl/images/0157/1902/14112.png)


