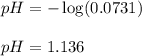Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
In a titration of 47.41 ml of 0.3764 m ammonia with 0.3838 m aqueous nitric acid, what is the ph of...
Questions


Biology, 24.03.2021 22:00



Mathematics, 24.03.2021 22:00

Mathematics, 24.03.2021 22:00


Mathematics, 24.03.2021 22:00

Mathematics, 24.03.2021 22:00




Chemistry, 24.03.2021 22:00

English, 24.03.2021 22:00


English, 24.03.2021 22:00

Biology, 24.03.2021 22:00

Mathematics, 24.03.2021 22:00


Chemistry, 24.03.2021 22:00

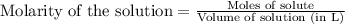
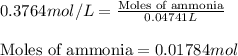
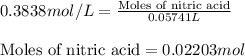
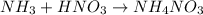
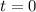 0.0178 0.022
0.0178 0.022![pH=-\log[H^+]](/tpl/images/0156/8638/cf945.png)
![[H^+]=\frac{0.0042mol}{0.05741L}=0.0731M](/tpl/images/0156/8638/dda8c.png)