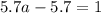Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
For the chemical equation so2(g)+no2(g)↽−−⇀so3(g)+no(g) the equilibrium constant at a certain temper...
Questions

Mathematics, 01.05.2021 14:00


Computers and Technology, 01.05.2021 14:00

Business, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00

Physics, 01.05.2021 14:00

Physics, 01.05.2021 14:00

Mathematics, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00

Mathematics, 01.05.2021 14:00

Arts, 01.05.2021 14:00



Mathematics, 01.05.2021 14:00

Health, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00

![Kc=\frac{[NO][SO_{3}]}{[NO_{2}][SO_{2}]}](/tpl/images/0156/8615/e939f.png)
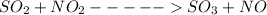
![Kc=3.80 = \frac{[1][1]}{[a-1][1.5]}](/tpl/images/0156/8615/9a395.png)