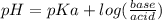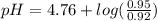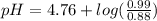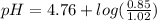Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the addition of the following species. (assume there is no change in volume.) (a) ph of starting buffer: (b) ph after addition of 0.040 mol naoh: (c) ph after further addition of 0.100 mol hcl:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the additio...
Questions

Geography, 27.02.2021 08:30


Biology, 27.02.2021 08:30


Chemistry, 27.02.2021 08:30


History, 27.02.2021 08:30


Chemistry, 27.02.2021 08:30

Mathematics, 27.02.2021 08:30

Mathematics, 27.02.2021 08:30

History, 27.02.2021 08:30

Arts, 27.02.2021 08:30

Spanish, 27.02.2021 08:30





Physics, 27.02.2021 08:30

Geography, 27.02.2021 08:30