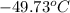Enter your answer in the provided box. a sample of sulfur hexafluoride gas occupies 9.53 l at 215°c. assuming that the pressure remains constant, what temperature (in °c) is needed to reduce the volume to 4.36 l? report your answer to the proper number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Enter your answer in the provided box. a sample of sulfur hexafluoride gas occupies 9.53 l at 215°c....
Questions

Biology, 29.06.2019 08:30

Social Studies, 29.06.2019 08:30

Social Studies, 29.06.2019 08:30


Chemistry, 29.06.2019 08:30




Biology, 29.06.2019 08:30



Mathematics, 29.06.2019 08:30


History, 29.06.2019 08:30


Mathematics, 29.06.2019 08:30


Chemistry, 29.06.2019 08:30


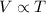
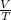 = constant
= constant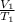 =
= 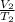
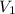 is 9.53 L,
is 9.53 L, 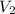 is 4.36 L,
is 4.36 L, 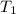 is
is 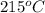 that is also equal to (215 + 273) K = 488 K.
that is also equal to (215 + 273) K = 488 K.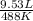 =
= 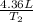
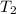 = 223.26 K
= 223.26 K