
Chemistry, 01.08.2019 01:30 ilianysacosta10
Ethanol (c 2h 6o(l), δh o f = -277.69 kj/mol) can be made by reaction of ethylene (c 2h 4(g) δh o f = 52.26 kj/mol) with water (δh o f = -285.83 kj/mol). what is the enthalpy of reaction for this process?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3


Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Ethanol (c 2h 6o(l), δh o f = -277.69 kj/mol) can be made by reaction of ethylene (c 2h 4(g) δh o f...
Questions




Chemistry, 16.05.2021 19:30

Mathematics, 16.05.2021 19:30

Mathematics, 16.05.2021 19:30

Mathematics, 16.05.2021 19:30

Mathematics, 16.05.2021 19:30

Mathematics, 16.05.2021 19:30


Mathematics, 16.05.2021 19:30



Mathematics, 16.05.2021 19:30

Advanced Placement (AP), 16.05.2021 19:30

Mathematics, 16.05.2021 19:30


History, 16.05.2021 19:30

Chemistry, 16.05.2021 19:30

Chemistry, 16.05.2021 19:30

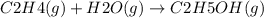
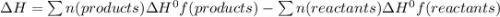
![\Delta H = 1\Delta H^{0}f(C2H6O)-[1\Delta H^{0}f(C2H4)+1\Delta H^{0}f(H2O)]](/tpl/images/0156/4062/38e6e.png)


