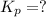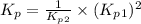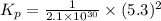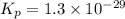Chemistry, 01.08.2019 01:20 christophergaudette0
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)kp=5.3 2no(g)⇌n2(g)+o2(g)kp=2.1×1030 use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: n2(g)+o2(g)+br2(g)⇌2nobr(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)...
Questions



Arts, 10.04.2021 21:20








Mathematics, 10.04.2021 21:30

Arts, 10.04.2021 21:30

Mathematics, 10.04.2021 21:30



English, 10.04.2021 21:30


World Languages, 10.04.2021 21:30


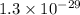
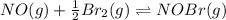 ;
; 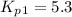
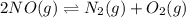 ;
; 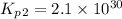
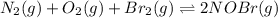 ;
;