
Chemistry, 01.08.2019 01:20 jademckinziemea
For the second-order reaction below, the initial concentration of reactant a is 0.24 m. if the rate constant for the reaction is 1.5 x10–2 m–1s–1, what is the concentration of a after 265 seconds? 2a --> b + crate = k[a]20.12 m0.19 m0.95 m4.0 m5.2 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
For the second-order reaction below, the initial concentration of reactant a is 0.24 m. if the rate...
Questions




English, 29.01.2020 06:55

Mathematics, 29.01.2020 06:55

Spanish, 29.01.2020 06:55

Mathematics, 29.01.2020 06:55

Mathematics, 29.01.2020 06:55

Social Studies, 29.01.2020 06:55

Mathematics, 29.01.2020 06:55



Social Studies, 29.01.2020 06:55



Computers and Technology, 29.01.2020 06:55

Mathematics, 29.01.2020 06:55


Mathematics, 29.01.2020 06:55

Chemistry, 29.01.2020 06:55

![\frac{1}{[A]}=\frac{1}{[A]_{0}}+kt](/tpl/images/0156/3924/a6d73.png)
![[A]_{0}](/tpl/images/0156/3924/48818.png) is intital concentration of A and k is rate constant.
is intital concentration of A and k is rate constant.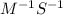 and t is 265 S
and t is 265 S![\frac{1}{[A]}=\frac{1}{0.24}+(0.015\times 265)](/tpl/images/0156/3924/91240.png)


