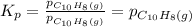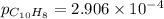Asample of solid naphthalene is introduced into an evacuated flask. use the data below to calculate the equilibrium vapor pressure of naphthalene (c10h8) in the flask at 35°c. hf (25c) gf (25c) c10h8(s) 78.5 kj/mol 201.6 kj/mol c10h8(g) 150.6 kj/mol 224.1 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Asample of solid naphthalene is introduced into an evacuated flask. use the data below to calculate...
Questions

History, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00


History, 06.07.2019 02:00

History, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00

History, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00

Biology, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00


History, 06.07.2019 02:00


English, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00

Chemistry, 06.07.2019 02:00

 atm.
atm.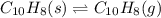
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0156/3886/45485.png)
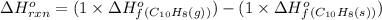
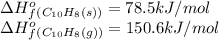
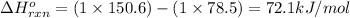
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f(product)]-\sum [n\times \Delta G^o_f(reactant)]](/tpl/images/0156/3886/b00b4.png)
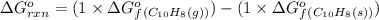
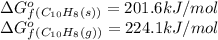
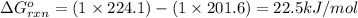
 (at 25°C) for given value of Gibbs free energy, we use the relation:
(at 25°C) for given value of Gibbs free energy, we use the relation: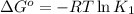
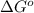 = Gibbs free energy = 22.5 kJ/mol = 22500 J/mol (Conversion factor: 1kJ = 1000J)
= Gibbs free energy = 22.5 kJ/mol = 22500 J/mol (Conversion factor: 1kJ = 1000J)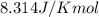
![25^oC=[273+25]K=298K](/tpl/images/0156/3886/0e82f.png)
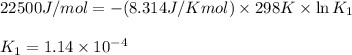
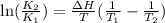
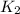 = Equilibrium constant at 35°C = ?
= Equilibrium constant at 35°C = ?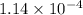
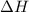 = Enthalpy change of the reaction = 72.1 kJ/mol = 72100 J
= Enthalpy change of the reaction = 72.1 kJ/mol = 72100 J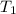 = Initial temperature =
= Initial temperature = 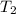 = Final temperature =
= Final temperature = ![35^oC=[273+35]K=308K](/tpl/images/0156/3886/b2088.png)
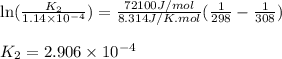
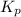 , which is:
, which is: