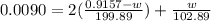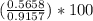Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
A0.9157-g mixture of cabr2 and nabr is dissolved in water, and agno3 is added to the solution to for...
Questions

Mathematics, 05.05.2022 17:40


Mathematics, 05.05.2022 17:40

Chemistry, 05.05.2022 17:50

English, 05.05.2022 18:00


Mathematics, 05.05.2022 18:20




Mathematics, 05.05.2022 19:40


Mathematics, 05.05.2022 19:40


History, 05.05.2022 19:50


History, 05.05.2022 20:10


Mathematics, 05.05.2022 20:10

History, 05.05.2022 20:30

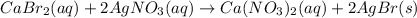
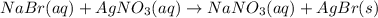
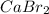 will be 0.9157 - w grams.
will be 0.9157 - w grams.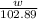
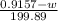
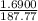 = 0.0090
= 0.0090