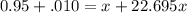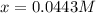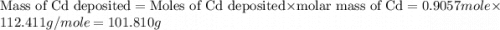Determine the mass of the metal at the cathode of the following galvanic cell when the cell is “dead” (at 298 k) assuming that the initial mass of both metal electrodes are 100 g and all solutions in the cell are exactly 1.0 l. fe | fe2+ (0.10 m) || cd2+ (0.95 m) | cd

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Determine the mass of the metal at the cathode of the following galvanic cell when the cell is “dead...
Questions





English, 04.10.2019 18:40


Mathematics, 04.10.2019 18:40

Mathematics, 04.10.2019 18:40

Mathematics, 04.10.2019 18:40

History, 04.10.2019 18:40


Mathematics, 04.10.2019 18:40




Social Studies, 04.10.2019 18:40




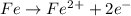
![E^0_{[Fe^{2+}/Fe]}=-0.44V](/tpl/images/0156/0778/59478.png)
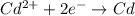
![E^0_{[Cd^{2+}/Cd]}=-0.40V](/tpl/images/0156/0778/d77be.png)
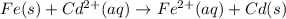
![E^0=E^0_{[Cd^{2+}/Cd]}-E^0_{[Fe^{2+}/Fe]}](/tpl/images/0156/0778/49ec3.png)
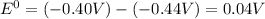
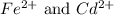 .
.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Fe^{2+}]}{[Cd^{2+}]}](/tpl/images/0156/0778/33164.png)
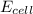 = emf of the cell = 0 (for dead cell)
= emf of the cell = 0 (for dead cell)![0=0.04-\frac{0.0592}{2}\log \frac{[Fe^{2+}]}{[Cd^{2+}]}](/tpl/images/0156/0778/3fae7.png)
![\frac{[Fe^{2+}]}{[Cd^{2+}]}=22.695](/tpl/images/0156/0778/c0251.png)
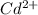 be, 'x'.
be, 'x'.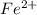 will be,
will be, ![22.695\times [Cd^{2+}]=22.695x](/tpl/images/0156/0778/44a3e.png)
![[Cd^{2+}]+[Fe^{2+}]=x+22.695x](/tpl/images/0156/0778/08dba.png)